INTRODUCTION
Periodontal regeneration is a multifactorial process and requires a multi-dependent sequence of biological events including cell-adhesion, migration, proliferation and differentiation.1 The ultimate goal of periodontal therapy is to regenerate the lost periodontal tissues caused by periodontitis.2 Various controlled clinical trials have demonstrated that some of the available grafting procedures may result in periodontal regeneration in intrabony defects, but complete and predictable reconstruction of periodontal tissues is still difficult to obtain.3 The reason is that periodontium, once damaged has a limited capacity for regeneration.4 The most positive outcome of periodontal regeneration procedures in intrabony defect has been achieved with a combination of bone graft and guided tissue regeneration.5, 6
The complex series of events associated with periodontal regeneration involves recruitment of locally derived progenitor cells subsequently differentiated into periodontal ligament forming cells, cementoblasts or bone forming osteoblasts. Therefore, the key to periodontal regeneration is to stimulate the progenitor cells to reoccupy the defects.
Growth factors are the vital mediators during this process which can induce the migration, attachment, proliferation and differentiation of periodontal progenitor cells. Platelet rich fibrin (PRF) may be considered as a second generation platelet concentrate, using simplified protocol, is a recently innovative growth factor delivery medium.
PRF allows to obtain fibrin mesh enriched with platelets and growth factors, from an anticoagulant free blood harvest without any artificial biochemical modification.7 The PRF clot forms a strong natural fibrin matrix which concentrates almost all the platelets and growth factors of the blood harvest and shows complex architectures as a healing matrix, including mechanical properties, which no other platelet concentrate can offer. In an article in 2011, Lekovic
In the present case report, we present the clinical and radiographic changes of a patient using L - PRF along with allograft as grafting material in the treatment of periodontal intrabony defect with endodontic involvement.
CASE REPORT
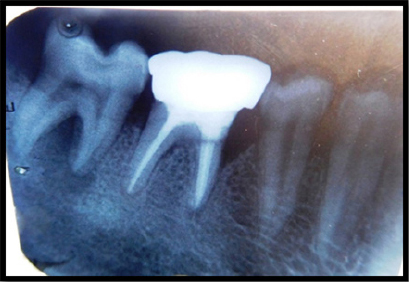
A 45-year-old systemically healthy female patient reported to the Department of Periodontology, Kamineni Institute of Dental Sciences, Narketpally, Telangana, with a chief complaint of food lodgement in right mandibular back tooth region of the jaw since 1 month. Patient underwent endodontic therapy in relation to #46 six months back. On periodontal examination, the probing depth on mesiobuccal aspect was 8mm. The patient also presented with pain on percussion in relation to #47 and a periapical radiograph was taken which on evaluation, presented with an intrabony defect extending up to middle third of the mesial root of right mandibular first molar (#46) (Figure 1). The diagnosis was made to be chronic generalized gingivitis with localized periodontitis in relation to #46
|
Figure 1: Preoperative radiograph showing an intrabony defect extending up to middle third of the mesial root of right mandibular first molar
Click here to view |
Pre Surgical Therapy
Initial therapy consisted of oral hygiene instructions, Scaling and root planning using ultrasonic instruments and curettes were performed. At 4 weeks following phase 1 therapy, a periodontal re-evaluation was performed to confirm the suitability of #46 tooth for this periodontal surgical procedure.
PRF Preparation
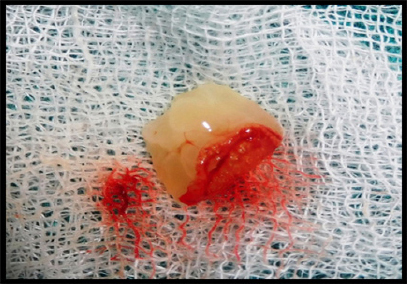
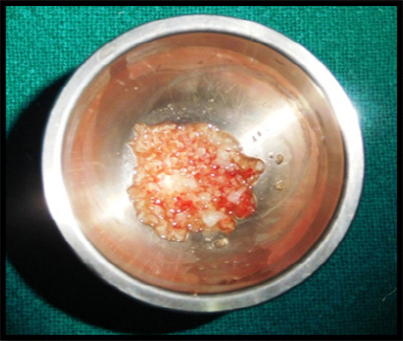
PRF preparation requires a centrifuge and collection kit including a 24 gauge needle and 9 ml blood collection tubes. After obtaining the informed consent, whole blood is drawn, into the tubes without anticoagulant and is immediately centrifuged at 3000 rpm for 10 min. The outcome is a fibrin clot containing platelets in the middle of the tube, between the red blood cell layer at the bottom and acellular plasma at the top. This clot is removed from the tube and the attached red blood cells scraped off and discarded. The PRF clot is then placed between two gauzes and pressed thereby squeezing out the fluids in the fibrin clot to obtain a matrix/ membrane which was later mixed with graft material (Figure 2 and 3).
|
Figure 2: PRF plug
Click here to view |
|
Figure 3: PRF with DFDBA
Click here to view |
Surgical Procedure
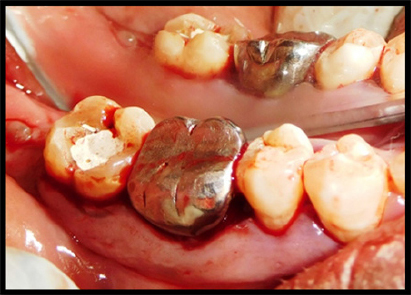
An intrasulcular incision was made on buccal and lingual aspect of the tooth of right mandibular teeth (#45, 46 and 47) (Figure 4). A full thickness mucoperiosteal flap was raised and inner surface of the flap was curetted to remove the granulation tissue. Root surfaces were thoroughly planned using hand instruments. The right mandibular first molar demonstrated mesial intrabony defect. After removing granulation tissue thoroughly, mesial intrabony defect was found to extend in buccal and apical aspect (Figure 5). The allograft (DFDBA - TATA MEMORIAL) with PRF was then condensed (Figure 6). The flap were repositioned to their pre- surgical levels and sutured with silk utilizing an interrupted technique (Figure 7). After the operation, the patient was prescribed systemic antibiotics (amoxicillin 500 mg tid, 3 days), non- steroidal anti-inflammatory drug (Hifenac D tid, 3) days) and 0.2% chlorhexidine rinse (twice a day for 4 weeks). Sutures were removed after 7 days. Clinical healing was normal. The patient was recalled at 1st week, 2ndweek, 1st month, 3rd and 6th month. Periapical intraoral radiographs were obtained from the periodontal defect site at baseline, 3 months and 6 months after surgery.
|
Figure 4: Intra sulcular incisions
Click here to view |
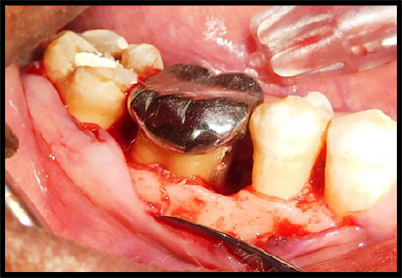
|
Figure 5: Defect in mesial aspect of #46
Click here to view |
|
Figure 6: PRF with DFDBA placement
Click here to view |
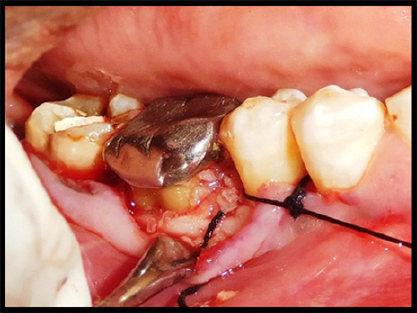
|
Figure 7: Sutures placed
Click here to view |
Results
In the present case report, the greater reduction in pocket depth was found after 6 months of the follow-up that is from 8 mm to 2mm which is the important clinical outcome for any periodontal regenerative procedures. Radiographs revealed significant bone fill in the intrabony defect compared with measurements at baseline (Figure 8).
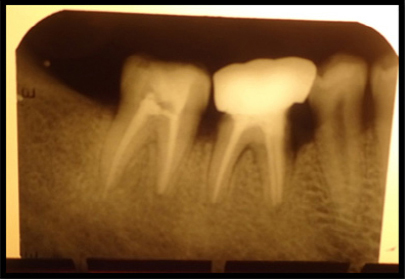
|
Figure 8: Post operative radiograph showing significant bone fill in the intrabony defect (6 months post operative).
Click here to view |
DISCUSSION
The present case report evaluated the clinical efficacy of L-PRF matrix in the treatment of a hard tissue defect. The grafted sites displayed rapid clinical healing, with good tissue contour, color and bone density. In our case, a significant reduction in PPD was found. Significant radiographic bone formation in the periodontal intrabony defect was also observed supporting the role of various growth factors present in the L-PRF matrix in accelerating the soft and hard tissue healing.
Despite of the fact that PRF is a denser and firmer agent than other biological preparations, such as PRP and enamel matrix derivative (EMD), it is still non-rigid to a degree that its space maintaining ability in periodontal defects is non-ideal. When platelets in a concentrated form are added to graft materials, a more predictable outcome is derived.9 DFDBA have been used in periodontal therapy for years together. It is known to have an osteogenic potential that is manifested by exposing bone morphogenic protein (BMPs) which presumably have the ability to induce host cells to differentiate into osteoblast. They have been successfully used to reconstruct intraosseous periodontal defects. In another study, PRF in combination in with bone mineral had the ability in increasing the regenerative effects in intrabony defects.8 For that reason allograft (DFDBA) was chosen hypothesizing that it could enhance the effect of PRF for tissue regeneration to occur.
Mechanism of Action of L- PRF:
The L-PRF preparation process creates a gel like fibrin matrix, which incorporates platelets, leukocyte, cytokines, and circulating stem cells. PRF progressively releases cytokines over a period of time, during fibrin matrix remodelling.10 Chang et al reported that PRF activation brings about expression of phosphorylated extracellular signal-regulated protein kinase (p-ERK) and osteoprotegrin (OPG) signifying its benefits for bone regeneration. Light and scanning electron microscopy showed leucocytes proliferating and interacting with osteoblasts leading to initiation in mineralization process.11 Moreover, L-PRF is capable of increasing osteoblast attachment, proliferation and simultaneously up-regulating collagen-related protein production all of which would effectively promote bone regeneration.
The matrix acts like a fibrin bandage that accelerates the healing of wound edges. It also provides a significant postoperative protection of the surgical site and seems to hasten the integration and remodeling of the grafted biomaterial.12
CONCLUSION
According to the results obtained in the present case report, it could be concluded that the positive clinical impact of additional application of PRF with allograft in treatment of periodontal intrabony defect is based on reduction in probing pocket depth, significant radiographic defect bonefill, improved patient comfort and early wound healing process. However, long-term, multicenter randomized, controlled clinical trial will be required to know its clinical and radiographic effect over bone regeneration.
