INTRODUCTION
Oral Sub-mucous Fibrosis is a chronic debilitating disease of the oral cavity characterized by inflammation and progressive fibrosis of the sub mucosal tissues.1 The clinical features of OSMF includes progressive inability to open the mouth (Trismus) due to oral fibrosis and scarring, pain and burning sensation upon consumption of spicy food stuffs, increased salivation, change of gustatory sensation, hearing loss due to stenosis of the eustachian tubes, dryness of the mouth, nasal tone to the voice, dysphagia (if esophagus is involved).2 The mainstay in the treatment of OSMF, is therefore concentrated on attempts to improve the mouth opening.3 Several medical and surgical therapies have been put forth with varied degree of success. Amongst the most widely accepted and frequently used surgical procedures are use of buccal fat pad or nasolabial flaps.4-7 In the experience of majority of surgeons it has been found that the rotation of nasolabial flaps in to the oral cavity and suturing these flaps in to the posterior most aspect of the defect is very difficult, in addition the difficulty is coupled by poor access and visibility in this area. These findings have prompted us to take up the present study of closure of the surgical defects in oral sub mucous fibrosis, posteriorly by buccal fat pad and anteriorly by nasolabial flaps. Till now there are studies either only on buccal fat pad or on nasolabial flaps.
MATERIALS AND METHODS
A total of twenty patients were selected with oral submucous fibrosis, staged III and IV according to Khanna and Andrade classification. Patients who were willing to quit the habits were included in the study. The exclusion criteria were patients with stage I and stage II oral sub-mucous fibrosis, below 18 years of age, uncontrolled systemic conditions and TMJ problems.
Surgical method
After informed consent the operation was performed under general anesthesia with nasal intubation. Incision was made from the angle of the mouth to the anterior tonsillar pillars down to the muscles, depending upon the location of the fibrotic bands (Figure 1) which restricted mouth opening (Figure 2). The mouth was forcefully open with a mouth opener to an acceptable range of approximately 35 mm. If intraoperative mouth opening is less than 35mm, then bilateral coronoidectomy or temporalis myotomy was done through the same incision. The buccal defects were then reconstructed by NasoLabial Flap anteriorly (Fig. 3, 4 and 5) and Buccal Fat Pad posteriorly (Figure 6). All the patients were followed up for 6 months.
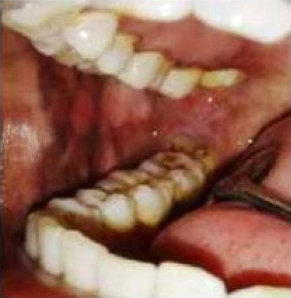
|
Figure 1: Preoperative picture showing fibrous bands
Click here to view |
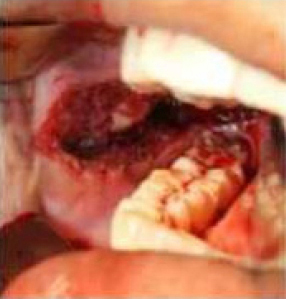
|
Figure 2: Intra-operative band excision
Click here to view |
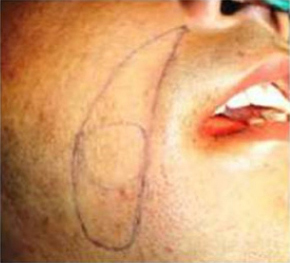
|
Figure 3: Naso labial flap marked
Click here to view |
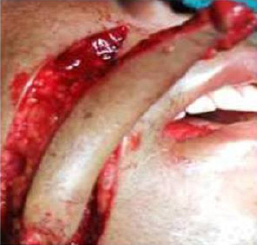
|
Figure 4: Nasolabial flap rotated intra orally
Click here to view |
|
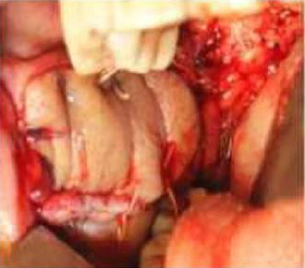
Figure 5: Nasolabial flap covering anterior region after excision
Click here to view |
|
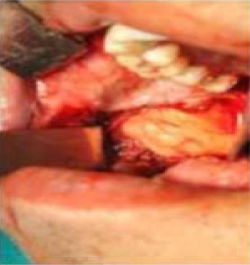
Figure 6: Buccal fat pad in the posterior region
Click here to view |
The following parameters were assessed. Duration of surgical procedure was measured from starting of the operative procedure till the surgical defect is closed. Mouth opening was assessed pre operatively and post operatively (Figure 7). Cheek flexibility was assessed clinically and areas with more suppleness were given ++ and areas where there was feeling of fibrous scar tissue or hardness of mucosa were given +. Inter commissural width was assessed using a scale and the distance between corners of the mouth on both the sides was measured. Time taken for the transposed nasolabial flaps to be fully covered with mucosa and complete epithelization of buccal fal pad was assessed through regular follow up. The esthetic outcome of the scar was assessed by observing the color and character of the scar and a score was given accordingly (Figure 8).
|
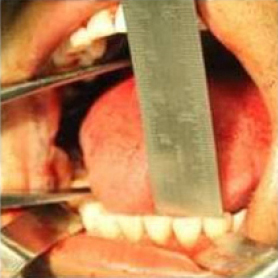
Figure 7: Post operative picture showing improved mouth opening
Click here to view |
|
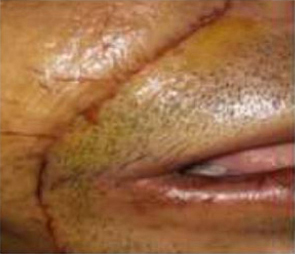
Figure 8: Post operative picture showing minimal scar in nasolabial region
Click here to view |
CHARACTER OF THE SCAR
01 - Noperceptible scar
02 - Visible but thin and linear scar
03 - Wide scar
04 - Hypertrophic scar or keloid
COLOR OF THE SCAR
01 - Noperceptible scar
02 - Normal skin color
03 - Red
04 - Hyperpigmented
05 - Hypopigmented
RESULTS
A total of 20 patients were operated with ages ranging from 25-66 years, with a pre-operative mouth opening from 6-17 mm (mean - 12.7 mm). Patients were assessed for mouth opening in the immediate post op, 15 days, 1st month, 3rd month and 6th month. The mean duration for epithelization of buccal fat pad was 4 weeks and for mucosilization of nasolabial flap was 5.65 months. Widening of the oral commissure was seen post surgery. The pre operative inter commissural distance ranged from 45-65 mm (mean 58.4mm) while the six months post operative values ranged from 49-69 mm (mean 62.4mm) with difference in the pre operative and post operative values ranging from 3-5 mm (mean of 4 mm).
Operating time was assessed for all the 20 patients and a mean operating time of 2hr 30.3min (150.3 min) was recorded.
DISCUSSION
Pindborg has defined Oral Submucous Fibrosis (OSMF) as “an insidious chronic disease affecting any part of the oral cavity and occasionally extending to the pharynx and esophagus, although, occasionally preceded by and/or associated vesicle formation. It is always associated with juxta- epithelial inflammatory reaction followed by fibroelastic changes in the lamina propria, with epithelial atrophy leading to stiffness of the oral mucosa causing trismus and difficulty in eating”.8
The common sites involved are labial mucosa, buccal mucosa, retromolar pads, soft palate and floor of the mouth. Fibrotic changes of the pharynx, esophagus and paratubal muscles of eustachian tubes had been reported. The fibrotic bands in the buccal mucosa run in vertical directions, in the soft palate the fibrous bands radiate from the pterygomandibular raphe or the anterior faucial pillars and in the lips circular bands can be felt around entire rima oris.9 The uvula is markedly involved in the late stages which shrinks and appears like a small bud.10 Patients complain of burning sensation, nasal regurgitation or nasal intonation and restricted mouth opening. It is associated with the betel nut, betel quid, tobacco and pan (a betel leaf folded around a mixture of tobacco, betel nut, lime, catechu and other flavouring agents) chewing habits including frequency (number of times per day), duration (years of consumption) and type (Gutkha, pan masala, Mava etc) was recorded. The areca nut (Areca catechu) is considered as the main etiologic factor causing OSMF, it generates free radicals and causes local immunosuppression. Seedat and Van Wyk in 1988 reported that OSMF also occurs in patients having no history of areca nut chewing.11-13 Furthermore it is reported that 7-13% of cases of OSF transform into squamous cell carcinoma according to Tilakaratne et al in 2006, although none of the patients in the present study have transformed into oral cancer. In the present study all the patients had a history of areca nut consumption for more than 8-10 years.14
Khanna JN and Andrade NN developed a group classification system for the surgical management of OSMF.15
Group I: Very early cases: Common symptom is burning sensation in the mouth, acute ulceration, recurrent stomatitis and not associated with mouth opening limitation.
Group II: Early cases: Buccal mucosa appears mottled and marble like, widespread sheets of fibrosis palpable, interincisal distance of 26 to 35 mm.
Group III: Moderately advanced cases: Trismus, interincisal distance of 15 to 25 mm, buccal mucosa appears pale, firmly attached to underlying tissues, atrophy of vermilion border, vertical fibrous bands palpable at the soft palate, pterygomandibular raphe and anterior faucial pillars.
Group IVA: Advanced cases: Severe trismus, interincisal distance of less than 15 mm, thickened faucial pillars, shrunken uvula, restricted tongue movement, presence of circular band around entire lip and mouth.
Group IVB: Advanced cases with presence of hyperkeratotic leukoplakia and/or squamous cell carcinoma.
The ideal goals of therapy include amelioration of the symptoms (burning sensation, restriction of mouth opening), and preventing further disease progression and malignant transformation. Various treatment modalities like medical, surgical are available to improve the patient condition. The medical management includes sub mucosal injection of steroids, placental extracts, hyaluronidase. This may give temporary relief but aggravates fibrosis because of the multiple needle insertion and irritation from the drug. The medical management is palliative therapy which is not going to reverse the condition completely. Surgical excision of bands is a preferred treatment procedure followed by reconstruction of the soft tissue defects after resection of fibrous bands.
In the present study, we considered Group III and IV for surgical treatment and Group I and II for medical treatment. The surgical aspect includes excision of OSMF bands followed by reconstruction with single stage bilateral nasolabial flap, which is covering the raw area created by excision of cheek mucosa anteriorly, and reconstruction with and buccal pad of fat posteriorly followed by supportive therapy with antioxidants.
In the present series of 20, all patients gave a positive history of chewing some form of betel nut or tobacco or a combination of the common form being roasted betel nuts. They had chief complaint of burning sensation upon eating spicy food, hot food or on intake of hot beverages and none of them complained of nasal regurgitation or nasal intonation. According to Khanna and Andrade’s14 grouping of OSMF based on clinical and histolopathogic features, 03 were group III while 17 belonged to group IVa.
All the patients were counseled pre-operatively regarding the treatment outcomes and quitting of the habit to avoid any chances of late post-surgical relapse. In all cases it was ensured that the patients stopped the habit at least three months prior surgery. They were given multivitamin therapy with antioxidants prior to surgery and continued even after the surgery at least for 6 months. Post operatively, all patients were advised to take bland diet.
Muhammad et al also recommended coronoidectomy in cases of trismus to achieve excellent mouth opening. All the patients in this study had coronoidectomy or temporalis myotomy as a routine procedure if the achieved mouth opening was less than 35 mm after excision of fibrous bands.
R.M. Borle et al has extensively used the extended NLF in the management of OSMF. Extended NLF is raised from the tip of NLF to the inferior border of mandible in the plane of the superficial musculoaponeurotic system from both terminal points to the region of the central pedicle. The pedicle is 1 cm lateral to the corner of mouth and the diameter of the pedicle is roughly 1 cm. The flap is transposed intraorally through a small transbuccal tunnel near the commissure of the mouth with no tension and sutured over intraoral defect.
The BFP was first identified by Heister in 1727. Egyedi (1977) first described the use of the BFP for closure of persistent oro-nasal or oro-antral communications.16 BFP harvested through an intraoral surgical site is simple, easy and has no donor site morbidity. On an average, the volume of BFP is 9.6 mL (range, 8.3-11.9 mL). Defects measuring up to 3-5 centimeters (cm) can be covered with BFP without compromising the blood supply was concluded in a study conducted by Stuzln et al.17
The present study was aimed at using both these versatile flaps in combination to reconstruct the defects following excision of fibrous bands in oral submucous fibrosis. In many occasions the authors have felt the approximation of the nasolabial flaps in retromolar trigone region or beyond is very difficult due to limited access. This technique is also very time consuming. Since we have to cover a large area of the defect, a large nasolabial flap needs to be raised which again leads to widening of the commissure. Since the versatility and usefulness of the buccal fat pad was already proved and particularly due to its proximity to posterior region, raising of the buccal fat pad and covering of the remaining defect with nasolabial flap raised on a small area, will reduce the efforts of the surgeon in reaching inaccessible areas also reducing the range of widening of commissure.
In our study, the age range was 25 to 60 years, and 11 were male and 9 were female, suggestive of high prevalence rates in males which is similar to male predominance, reported by various authors.
All the patients presented with a range of pre operative mouth opening from 6 to17 mm (mean - 12.7 mm). Patients were assessed for six months post operatively. Post operatively all the cases had a mean mouth opening of 43.95 mm ranging from 35 to 48 mm. Borle et al observed that interincisal mouth opening improved significantly from a mean of 14mm (range 3-23) to a mean of 41mm (range 23-55) using NLF.7 Sharma et al also used BFP for reconstruction of the defect in Stage III and IV OSMF patients.18 The mouth opening after 1-year follow-up was 35 mm in stage III group and 31.76 mm in stage IV group. Vigorous postoperative physiotherapy was necessary to maintain the postoperative mouth opening achieved intraoperatively.
In the present study, epithelization of BFP was seen with a mean of 4 weeks duration. It was 3 weeks in cases - 1, 5, 7, 10, 15 and 18; 4 weeks in cases - 3, 4, 6, 8, 9, 11, 14, 16 and 19; 5 weeks in cases - 12, 13 and 20; 6 weeks in case no - 17. However Tideman et al first described the use of the uncovered BFP as a pedicled graft in the mouth, and showed that epithelization occurred within 2-3 weeks, leaving a good mucosal surface.19
Donor site morbidity is negligible with the use of NLF. It effectively covers both posterior and anterior defect of fibrotomy in cases of OSMF. Extraoral scar, infection, minor or major flap necrosis, wound dehiscence do occur in a small number of patients as stated by Ioannides and Fossion.20 Asymmetry at the level of the nasolabial fold is noted if proper suturing is not done. The most common complication with the use of NLF was the extraoral scar and intraoral growth of hairs over the flap. Garatea et al recommended the use of a cheek rotation flap for aesthetic postoperative scar.21 In the present study the post operative extraoral scars were hidden in the NLF. Out of the 20 patients, in 2 cases the character of the scar was not perceptible. In 16 cases thin and linear scar was visible. In two cases scar is wide but acceptable. The color of the scar merged with normal skin color in 18 cases and not perceptible in 2 cases. Intra oral hair growth was seen in all the male patients that reduced significantly after 6 months post operatively. The preoperative mean inter commissural distance was 58.4 mm which extended to 62.4 mm after 6 months postoperatively, with an increase by 4.00 mm. The mean duration of surgery was 2 hours 30 minutes which was less when compared with the use of NLF alone. The combined technique has considerably reduced operating time. This was due to the ease of access and ease of adaptation of the BFP to the posterior aspect of the defect.
Possible intra operative complications included damage to facial vessels, stenson’s duct and branches of facial nerve. However we have not encountered these in any of the 20 cases. The extent of the defect that was replaced by NLF along with BFP was much wider with minimal tension in comparison with buccal fat pad or nasolabial flap alone. The size of the defect that can be covered by buccal fat pad was on an average of 3-5 cm and nasolabial flap can cover the defect upto 9 cm. Though nasolabial flap can cover a large area up to anterior faucial pillars, adaptation of this flap to this area is difficult and in the experience of many surgeons, the posterior teeth (1st and 2nd molars) were sacrificed to facilitate its suturing in posterior most aspect. By combining both the flaps in this study, the suturing of buccal fat pad was accessible and easy in posterior region. So, this procedure facilitates easy suturing eliminating the need for extracting the posterior teeth.
Regarding cheek flexibility, the cheeks were soft and elastic in areas covered by nasolabial flap 6 months postoperatively where as in areas covered by buccal fat pad it was slight fibrous and less elastic. But this did not hamper the inter incisal opening.
THE HEALING PATTERNS OF THE FLAPS WERE AS FOLLOWS:-
HEALING OF NASOLABIAL FLAP: During 1st Week- Close apposition of the edges of flap and the oral mucosa. All the sutures were intact without breaking down of wound, 1st month - Better fusion of edges, 3rd month - The graft intra oral site predominantly seen still in the form of keratinized epithelium with minute pinkish areas, 4th month - The grafted intra oral site shows mixed appearance of pinkish mucosa like areas mixed with keratinized mucosa, 6th month - Minor patchy areas of keratinized epithelium seen with no differentiation of the borders of the flap with the mucosa and a predominantly well healed pinkish mucosa like areas were seen.
HEALING OF BUCCAL FAT PAD: During 1st Week - loose apposition of the flap with the edges of the oral mucosa. Hence vigorous oral prophylaxis and irrigations were avoided during this period, 2nd Week - raw areas of the defect covered by the grafted flap. Minor sloughing of the graft superficially observed, 3rd Week - the grafted flap appeared healthier, gaining pinkish colour both from center and edges. At this stage all unresorbed sutures were removed, 4th Week- Complete epithelization of the fat was nearly complete, the fat was blended with normal mucosa with softening and elasticity of the transposed mucosa improved.
CONCLUSION
The advantages we have found while using the NLF and BFP together are that it was a relatively easy procedure with proximity of the donor sites, rich vascular supply of the flaps, versatility in design, easy flap elevation and causing minimal esthetic deformity. Amount of the defect that could be satisfactorily closed by the use of NLF along with BFP is greater with minimal tension when compared with the NLF or BFP alone. Widening of oral commissure is less when compared with NLF alone. Scar perception is less when compared with NLF alone. There is reduced operating time with less intraoral scar retraction when compared with BFP alone, so more chances of maintaining the achieved mouth opening.
