INTRODUCTION
Regeneration requires an orchestrated sequence of biologic events, such as cell migration, adherence, growth, and differentiation, to have the potential to increase the success and predictability of regenerative procedures. Platelets play a crucial role in hemostasis and wound healing. The á-granules of platelets release platelet-derived growth factor (PDGF), transforming growth factor (TGFb), and insulin-like growth factor (IGF-I). During activation, á granules fuses with platelet cell membrane releases growth factors. These growth factors bind to transmembrane receptors of target cells (Osteoblasts, fibroblasts, endothelial cells, and epithelial cells) leads to expression of various genes resulting cell proliferation, collagen synthesis and osteoid formation.1
Autologus preparations are basically Fibrin sealants (FS), platelet rich plasma (PRP) and Platelet rich fibrin (PRF).
A physiological enriched fibrin complex matrix (PRF) releases growth factors in a controlled manner for longtime when compared to fibrin glue enriched with cytokines.
The preparation of PRF is very simple. A blood sample is taken without anticoagulant in 10ml tube then centrifuged in a table centrifuge at 2700 rpm for 12 mins. In the absence of anticoagulant, platelet activation and fibrin polymerization are triggered in a natural manner immediately.4 Fibrinogen is at first concentrated in the upper part of the tube, until the effect of the circulating thrombin transforms it into a fibrin network. The result is a fibrin clot containing the platelets located in the middle of the tube, just between the red blood cell layer at the bottom and acellular plasma at the top
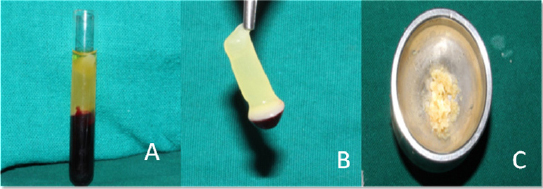
|
Figure 1: a) Platelet -rich fibrin in test tube; b) Platelet-rich fibrin; c) PRF mixed with bone graft.
Click here to view |
It contains cytokines such as IL-1, -4, -6, and growth factors such as Transforming Growth Factor beta 1 (TGF-â1), Platelet Derived Growth Factor (PDGF), and Vascular Endothelial Growth Factor (VEGF).5 The combination of fibrins and cytokines within PRF becomes a powerful scaffold with an integrated reservoir of growth factors for tissue regeneration. The fibrin matrix in PRF acts as natural guide for angiogenesis, natural support to immunity and guides the coverage of wounds
CLINICAL IMPLICATIONS OF PRF: 3-5
-
In sinus lift procedures
-
Socket preservations
-
Intrabony defects with or without bone grafts
(Figure 1c) -
PRF membrane has been used for gingival recession coverage with coronally advanced or lateral pedicle flap for multiple and single recession respectively
-
Endo perio lesions/Furcation defects
ADVANTAGES OF PRF OVER PRP: 3
-
No need of addition of bovine thrombin or other anticoagulants so it is completely safe.
-
Standard production protocol.
LIMITATIONS OF PRF: 5
-
As it is produced from autologus blood in limited quantities, limits the systemic utilization in general surgery or extensive surgical procedures.
-
PRF tissue banks are unfeasible. The fibrin matrix contains all the circulating immune cells and all the highly antigenic plasmatic molecules. That is why PRF membranes are totally specific to the donor and cannot constitute an allogenic graft tissue
TITANIUM-PREPARED PLATELET-RICH FIBRIN (T-PRF)
Some authors are worried about glass- evacuated blood collection tubes with silica particles as these particles may cause health hazards.5 Only small fraction of these silica particles are sediment ting with red blood cells. Majority of silica particles suspends in a buffy coat so that these particles reach to patient when these product is used for treatment.6 Although this is not practically concluded (the architecture of L-PRF change with type of material used for its preparation), some of the authors used more biocompatible material titanium for PRF preparation

|
Figure 2: Titanium test tubes for T-PRF preparation
Click here to view |
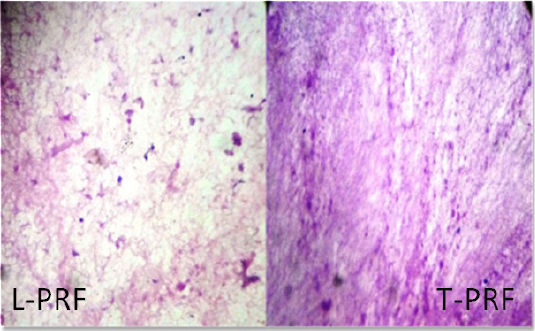
|
Figure 3: Histological analysis shows more number of fibroblasts and thicker fibrin structure in T-PRF when compared with L-PRF
Click here to view |
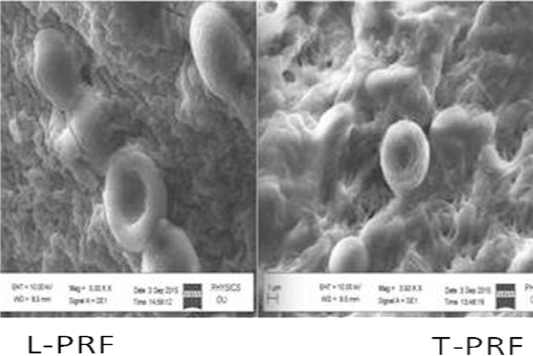
|
Figure 4: SEM analysis shows more woven and thicker fibrin structure in T-PRF when compared with L-PRF
Click here to view |
RATIONALE BEHIND INVENTION OF A-PRF
-
Monocytes releases higher concentration of growth factors.
-
Decreases centrifugation rpm and increased time gave enhanced neutrophil granulocytes in the distal part ( yellow color) of the clot
-
Neutrophil granulocytes increases differentiation of monocytes
PRF clots formed with A-PRF centrifugation protocol showed a loose structure with more interfibrous space, and more cells in distal part of fibrin clot
PRF in terms of absorption time in body and clinical advantage over L-PRF.9
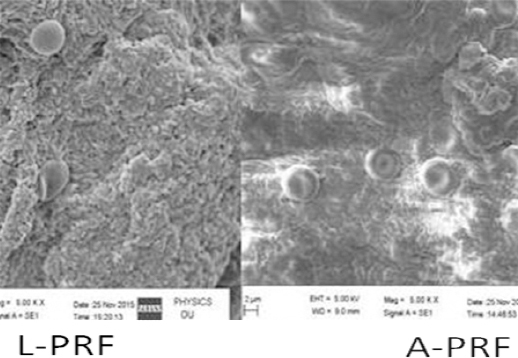
|
Figure 5: SEM analysis shows more a loose structure with more interfibrous space in A-PRF when compared with L-PRF
Click here to view |
INJECT ABLE PRF (I-PRF)
The centrifugation protocol of A-PRF is 700 rpm 3-4 mins, Differ from Standard PRF Which is 2700 rpm 12 mins. Un coated tube uses for preparation of A- PRF and I-PRF.10
RATIONALE BEHIND INVENTION OF A-PRF
-
Monocytes releases higher concentration of growth factors.
-
Decreases centrifugation rpm and increased time gave enhanced neutrophil granulocytes in the distal part (yellow color) of the clot
-
Neutrophil granulocytes increases differentiation of monocytes
This liquid PRF mix with allo graft results steaky bone which uses for augmentation procedures around implants.
|
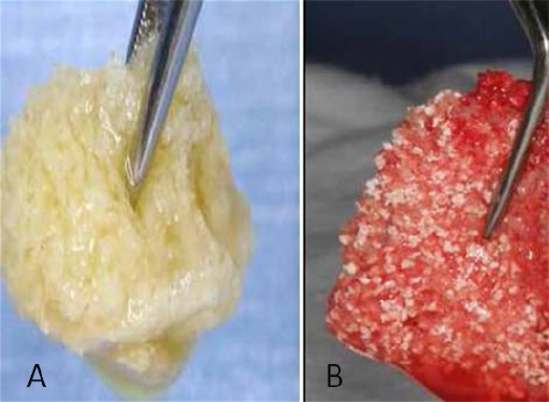
Figure 6: A) Mixing of AFG (Autologus fibrin glue) to allograft or to mixture of allograft and xenograft produces Yellow sticky bone; B) Addition of exudates from CGF (Concentrated growth factors) to the mixture of AFG and allograft or to the mixture of AFG and allograft and xenograft combination produces red color sticky bone formation
Click here to view |
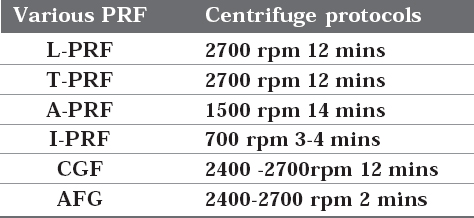
Brief Summary on Centrifugation Protocol of Various PRF
|
Click here to view |
CONCLUSION
The use of PRF shows positive edge over conventional treatments in the process of regeneration of diseased tissue as they release various polypeptide growth factors. Despite the evidence of clinical advantage of these preparations, evidence of their beneficial effects is still lacking. Hence there is a requirement for the justification of their widespread use. Additional studies will be required for the determining the full effect of these preparations. They will be welcomed for use by the Periodontist, as long as they provide consistent benefit.
